Statics: Snapshots of the Ventilatory Pump
Alveoli: Distending Pressures and the Stability of Lung Units
To this point, we have been examining the lung at a "macro" level. We have reviewed the properties of the chest wall and lungs as well as the interactions between these structures that help us to understand the balance of forces that guides inhalation and exhalation. These forces determine the lung volumes FRC, TLC, and RV. A more complete understanding of these forces, however, depends on an examination of the lung at a "micro" level; we must look at the properties of the alveolus and how it behaves during the respiratory cycle.
Transmural and Transpulmonary Pressure ↑
Earlier in the chapter, we introduced the concept of transmural pressure. We will now extend that discussion in the context of the alveolus. Transmural pressure is a general term for pressure across the wall of an object (transmural means "across the wall") and can be described by the following equation:
A flexible container or object expands if there is a positive transmural pressure (pressure greater inside than outside the object) and gets smaller with a negative transmural pressure. For this reason, a positive transmural pressure is sometimes referred to as a "distending" pressure. (Remember, one can describe the transmural pressure of the alveolus, the airway, or for that matter, the right ventricle of the heart. The term is general and can apply to any structure with a wall.)
For the alveolus, the pressure inside is the alveolar pressure, Palv, and the pressure outside is the pleural pressure, Ppl. Therefore, transmural pressure for the alveolus can be written as follows:
The pressure in the alveolus is a result of two forces: the elastic recoil of the lung (a collapsing force, Pel)), and the forces surrounding the lung (the pleural pressure). Thus, alveolar pressure is always greater than pleural pressure.
Substituting this expression for Palv into the previous equation, the transmural pressure of the alveolus can be expressed in terms of elastic recoil pressure:
Thus, under static conditions, the distending pressure of the lungs—the pressure that determines the volume of the lung at that moment in time—is the same as the elastic recoil pressure.
(Clinically, you will hear the term transpulmonary pressure used. This is the difference between the pressure at the mouth [taken to be the 0 reference point] and the pleural pressure. Transpulmonary pressure is used instead of alveolar transmural pressure because there is no practical way to measure the alveolar pressure.)
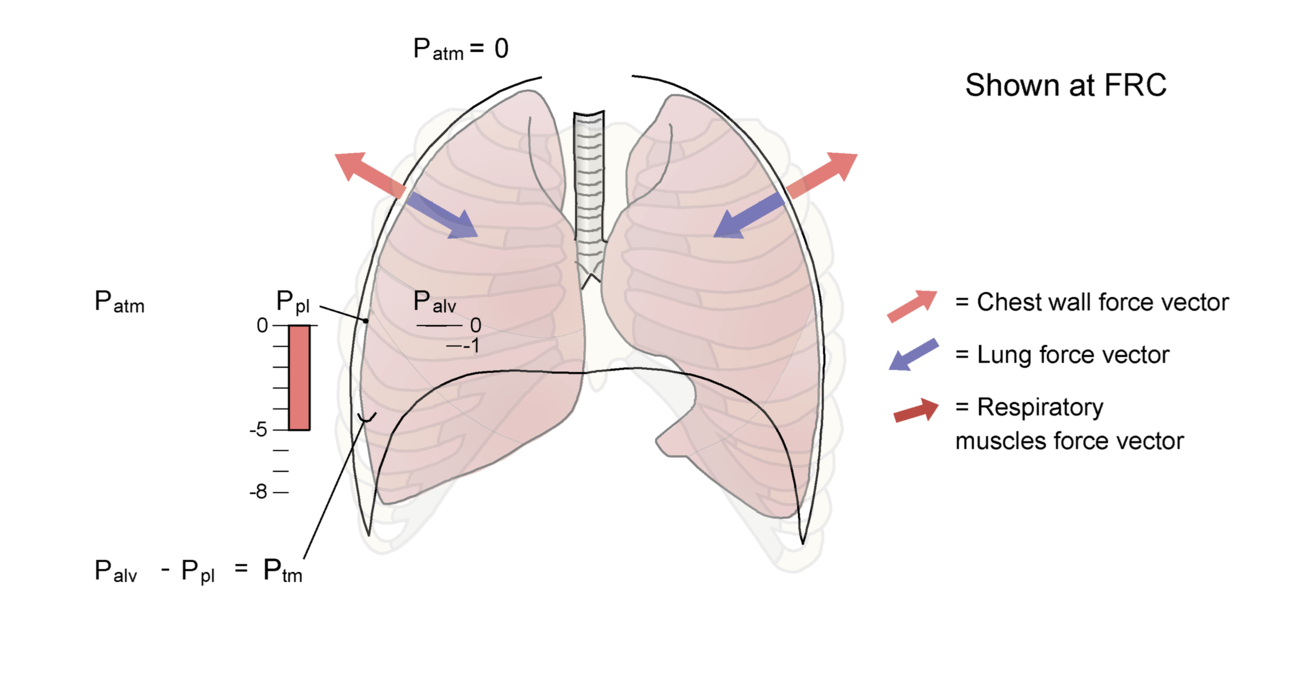
At FRC, when no ventilatory muscles are activated, the pleural pressure reflects the interaction between the tendency of the lungs to collapse (elastic recoil) and the tendency of the chest wall, at this volume, to spring out or expand. This creates a small negative pressure in the pleura, approximately -3 to -5 cm H2O. Because the alveolar pressure at FRC is 0 (this is the transition between expiration and inspiration, so there is no flow), the transmural pressure and the elastic recoil pressure must be between 3 and 5 cm H2O (equal and opposite to the pleural pressure) at this volume (Fig. 3-10A). A greater transmural pressure equates with a greater lung volume and greater elastic recoil.
Figure 3-10 Setting the system in motion
A The lungs and chest wall at functional residual capacity (FRC). Note that the force exerted by the lungs inward (the elastic recoil of the lungs) is equal in magnitude but opposite in direction to the outward recoil force exerted by the chest wall. Under static conditions, the alveolar pressure is equal to pressure at the airway opening (mouth) or atmospheric pressure (Patm), which is 0. Pleural pressure at FRC is shown as -5 cm H2O. B We now set the system in motion by activating the inspiratory muscles (note the new force vector) and moving air into the lungs. The chest wall has moved outward because of the action of the inspiratory muscles, and pleural pressure has decreased to approximately -7 cm H2O. Pressure in the alveolus has decreased to -1 cm H2O, which establishes a pressure gradient between the airway opening and the alveolus, leading to flow of gas into the lungs. Alveolar pressure has not changed as much as pleural pressure because of the effect of other factors such as airway resistance, tissue resistance, and the acceleration of gas as flow is initiated (see Chapter 4). The lung is now at a higher volume, and the elastic recoil forces are greater than at FRC. The chest wall has moved closer to its resting position, so the outward recoil forces of the chest wall have diminished. Also see Animated Figure 3-10.
A
B
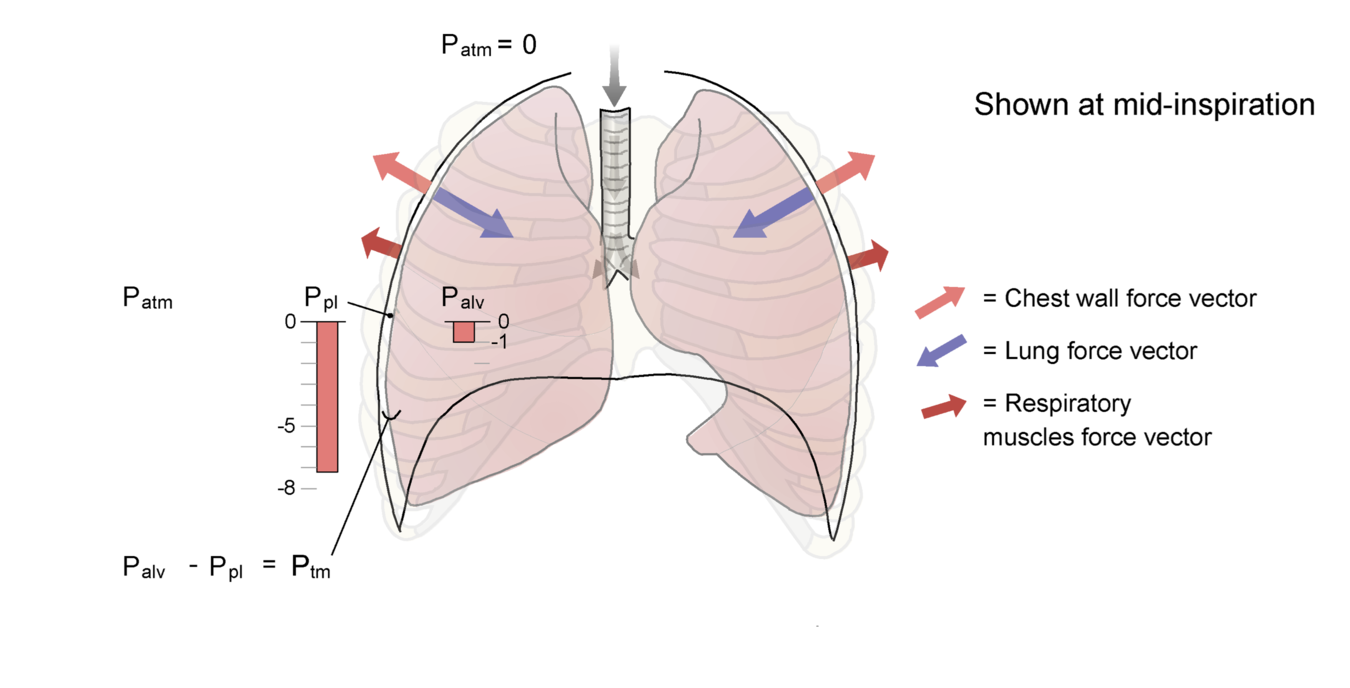
Let's begin to set the system in motion. The inspiratory muscles are activated, pulling the chest wall outward, and pleural pressure becomes more negative. This negative pressure is transmitted to the alveolus. Alveolar pressure, which had been 0 an instant before, is now negative as well (Palv = Pel + Ppl)). There is now a pressure differential between the airway opening and the alveolus, and air flows into the lung (Fig. 3-10B shows a snapshot of an intermediate point in the inspiration).
Use Animated Figure 3-10 to view the sequence delineated above and pay special attention to how the alveolar and pleural pressures change during the inspiration. Transmural pressure across the alveolus is indicated in the diagram. You can use the slider to move slowly back and forth through the animation and observe the pressure changes. Note how the alveolar pressure becomes negative, causing airflow, and then returns to 0 as we reach static conditions again at the end of inspiration.
Alveolar Stability ↑
The alveolus is the primary site of gas exchange in the lungs. For gas exchange to occur, the alveolus must remain open. The physiology of alveolar stability is one of the most important and difficult areas of respiratory physiology. An understanding of this topic is essential for the effective management of patients with respiratory distress syndrome, a condition that leads to alveolar collapse and severe problems related to the low blood oxygen levels that result when lung units no longer contain air.
The Alveolus as a Bubble
As we begin our exploration of the forces that influence the stability of the alveolus, let us consider a soap bubble. The bubble consists of a spherical film of liquid soap surrounding gas; thus, there is a gas-liquid, or air-liquid, interface. This interface or surface gives rise to surface tension, which is defined as the force with which a surface contracts per unit length of surface and has the units (dynes/cm2). A molecule within a liquid is surrounded on all sides by other similar molecules, which exert force on it. Because of the symmetry of the arrangement of the molecules, there is no net molecular movement. In contrast, at the surface of the liquid, the molecules interact with other liquid molecules in some directions (e.g., laterally in the surface of the liquid and with molecules in the subsurface layers) but not in other directions (toward the air interface). The intermolecular forces across the surface, between the liquid and gas molecules, are much weaker than the forces between molecules within the liquid. This imbalance of intermolecular forces at the surface, distinct from the uniform field that would be applied to a given molecule located in a subsurface layer, causes the molecules at the surface to be drawn toward the interior of the liquid. The net effect of this arrangement is that the surface tends to contract; the strength of that contraction is the surface tension (Fig. 3-11).
These forces allow a leaf to float on the surface of a quiet pond. Surface tension causes the surface to assume the smallest area possible, that is, the molecules at the surface come as close together as possible as they are drawn toward the subsurface molecules. In the case of a sphere such as soap bubble, this force is translated to a three-dimensional object. The tendency for the surface to assume the smallest possible area—in this case, the smallest area of a sphere—produces a force that, if unopposed, leads to the collapse of the sphere.
Law of Laplace
If surface tension causes the sphere to assume the smallest radius possible, what would stop the soap bubble from collapsing? To answer this question, we must consider the law of Laplace, which can be stated as follows:
where
Alveoli may be thought of as microscopic, spherically shaped objects lined with a thin film of liquid. As the lungs inflate, the alveoli increase in radius; as the lungs deflate, the radius diminishes. Because of the weight of the lungs and differences in transpulmonary pressure across the alveoli in varying regions of the lungs (see Chapter 4), alveoli do not increase and decrease in size uniformly throughout the lungs over the course of the respiratory cycle. During exhalation, the smallest alveoli, which tend to be in the more dependent or lower regions of the lungs, in a gravitational sense, are most prone to collapse.
The surface tension of water is constant across the entire surface and not dependent on the area of the air-liquid interface. Thus, in keeping with the law of Laplace, bubbles with a small radius must have a greater pressure within them than bubbles with a large radius.
Because gas flows from regions of high pressure to regions of lower pressure, the small bubbles empty into the larger bubbles. Use Animated Figure 3-12 to view how bubbles with different sizes and pressures evolve over time when connected together. If the alveoli behaved as soap bubbles do, small alveoli would collapse into larger alveoli, and gas exchange (i.e., the ability to get oxygen into the blood and to remove carbon dioxide from the blood) would be seriously deranged. Given the variable sizes of alveoli and their interconnectedness via the airways, something must reduce the surface tension forces as alveoli decrease in size during exhalation, or segments of our lungs would collapse repeatedly with each breath. That "something" provided by the type II pneumocyte is a detergent called surfactant.
Surfactant
Detergents are substances with a polar and a nonpolar end. The molecules of a detergent are interposed between the polar molecules of water. Recall from our discussion of surface tension that the molecules of a liquid at an air-liquid interface are affected by particularly strong intermolecular forces. The interposition of a detergent between the molecules at the surface layer diminishes those forces and, thereby, reduces surface tension. Typically, a detergent's effect on surface tension is constant regardless of the surface area into which it is dispersed.
Surfactant, known chemically as dipalmitoylphosphatidyl choline, has the lowest surface tension of any biological substance ever measured. The type II pneumocytes lining the lung produce surfactant, which enters into the liquid lining the alveolus. Unlike most detergents, surfactant's effect is variable at different surface areas, and it is further dependent on the direction of change in the surface area (i.e., whether the alveolus is being inflated or deflated).
During lung inflation, molecules of surfactant appear to move into the layer of molecules at the surface of the liquid lining the alveolus (i.e., at the air-liquid interface). This process reduces surface tension and makes it easier to expand the lung. In this sense, surface tension plays a role, along with the elasticity of the lung tissue, in determining the compliance of the lung. As surface tension is reduced, compliance increases. As lung volumes further increase, the density of surfactant molecules in the surface layer remains constant, and the elastic properties of the lung reassert themselves as the main determinant of compliance.
During deflation of the lung, the density of surfactant molecules increases as surface area declines. This leads to a decrease in surface tension. If surface tension declines, pressure in the alveolus may decrease without leading to a significant reduction in the size of the alveolus (recall the law of Laplace). With further exhalation, the alveolar radius diminishes, and some of the surfactant molecules are extruded from the surface layer to form subsurface collections, or micelles, while others are degraded. The molecules in the micelles may be recruited to enter the surface layer during the subsequent inhalation. The density of surfactant molecules in the surface layer during this second phase of exhalation appears to be constant. As the size of the alveoli and the surface area continue to decrease during the final stage of exhalation, surfactant density reaches a maximum. Further decreases in radius of the alveolus are not associated with greater decreases in surface tension.
Viewing the lung in its entirety, the effect of surfactant can be viewed as increasing pulmonary compliance, that is, the change in pressure required to achieve a given change in lung volume is less in the presence of surfactant than if it did not exist. In addition, during exhalation, surfactant, by reducing surface tension, helps to prevent alveolar collapse and derangements in gas exchange that result when alveoli have no air within them.
A third effect of surfactant is to minimize transudation of fluid from the pulmonary capillaries that line the alveoli. The forces associated with surface tension have the effect of reducing the hydrostatic pressure in the tissue around the capillaries; in a sense, the surface tension tends to "suck" fluid from the capillaries and facilitates accumulation of fluid in the alveolar space. Thus, the reduction in surface tension associated with the presence of surfactant helps to keep the alveoli dry.
Hysteresis
Consider the following experiment. We take a sheep lung in vitro, fill it with liquid, and inflate it and deflate it by moving liquid into and out of the lung. Now we remove the liquid (at least most of the liquid from the airways and alveoli, leaving only a thin liquid layer within the alveoli themselves) and inflate and deflate the lung by moving air into and out of the lung. Plot the pressure-volume curves for each condition. Will they look the same or different?
As you can see in Figure 3-13, when the lung is filled with liquid, the inflation and deflation curves can be superimposed. In contrast, when the lung is inflated with air, the two curves become separated; the inflation curve is shifted to the right of the deflation curve. This characteristic of air-filled lungs, the separation of the inflation and deflation limbs of the pressure-volume curves, is known as hysteresis. Because the only difference between the two conditions is the creation of an air-liquid interface, we hypothesize that hysteresis is a reflection of surface forces that accompany such an interface (i.e., surface tension) and the role of surfactant in modulating surface tension.
At the alveolar level, we discussed how surfactant molecules, newly produced by type II pneumocytes or drawn from subsurface micelles, enter into the liquid surface layer after the initiation of inhalation and begin to reduce surface tension. Thus, the initial part of the inflation curve is relatively flat; surface tension is high and compliance is low. As the density of surfactant in the surface layer increases, surface tension decreases and compliance increases; the slope of the curve becomes steeper. Eventually, as the volume of the alveolus continues to increase, surfactant density remains constant and the elastic recoil forces of the lung become the primary explanation for changes in compliance. At high lung volumes, the elastic recoil of the lung increases, compliance decreases, and the slope of the curve begins to flatten.
During deflation, the density of surfactant molecules in the surface layer rapidly increases; surface tension decreases, and the initial portions of the deflation curve are fairly flat. In essence, alveolar pressure decreases, but because of the concomitant decrease in surface tension, there is little change in volume. With further deflation, some surfactant molecules are extruded from the surface layer, and the density of surfactant remains relatively constant. The compliance of the lung, reflecting effects of elastic recoil and surface tension, is also constant during this phase of exhalation. Surfactant appears to have a greater effect on compliance of the lung during exhalation than during inhalation, probably because of the time course for surfactant molecules to enter and exit the surface layer. As the lung is expanding, there is some delay in the movement of molecules of surfactant from the subsurface to the surface. Thus, for any given lung volume, the compliance of the lung is less during inhalation than during exhalation. If we view the pressure-volume curves and hysteresis from the perspective of the entire lung rather than at the level of the alveolus, another factor must be considered. At the end of exhalation, even with surfactant present, some alveoli collapse, particularly at the bases of the lung, where transpulmonary pressure may be negative (pressure outside the alveolus greater than pressure inside the alveolus). To reopen these collapsed alveoli at the beginning of the next inspiration, or recruit them to participate in gas exchange (remember that a collapsed, airless alveolus cannot provide oxygen to or eliminate carbon dioxide from the pulmonary capillaries), requires a relatively high change in pressure. This pressure, termed the critical opening pressure, may be as high as 20 to 30 cm H2O in animal experiments. Thus, the initial portion of the inflation curve, with its fairly low slope, may partly reflect the recruitment of collapsed alveoli.
One other factor may contribute to the observation of hysteresis in the lungs. When one inflates the lung and holds the lung at the higher volume for several seconds, the elastic recoil forces appear to diminish slightly, a phenomenon called stress relaxation. Conversely, after deflation of the lung, the recoil forces increase; this process is called stress recovery. This phenomenon, called stress adaptation, is believed to be caused by the viscoelastic properties of smooth muscle and connective tissue in the lung but, relative to the effect of surface forces discussed above, plays a small role in hysteresis.